Zoll Medical Introduces Two New AEDS in the UK
Tuesday, 16 August, 2016
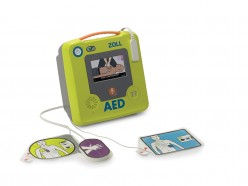
ZOLL AED 3™

ZOLL AED 3™ BLS
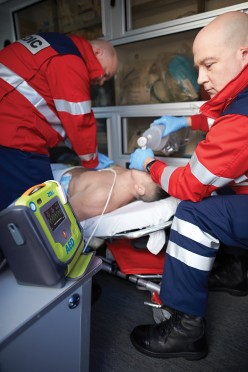
ZOLL AED 3—Even Better Support for Rescuers
12th August, 2016—Cheshire, UK—ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today that it is expanding its existing product line of automated external defibrillators (AEDs) in the UK with the introduction of the ZOLL AED 3™ and the ZOLL AED 3™ BLS (basic life support). www.zoll.com/uk/aed3
These new AEDs build on ZOLL’s legacy of introducing new technology to the AED market to help improve outcomes for cardiac arrest patients. Enhanced Real CPR Help® gives rescuers the power to know when they are providing high-quality chest compressions. ZOLL has also introduced the world’s first 5-year universal electrode for both adult and paediatric patients, further improving cost of ownership. Every ZOLL AED 3 comes with Programme Management Onboard™, which notifies users immediately if the device fails a self-test, or if the battery is due to be replaced, ensuring the device is ready to be used.
The ZOLL AED 3 BLS model is designed specifically for the needs of first responders with the CPR Dashboard™ and the ability to deliver the patient record directly to health care providers.
“We are extremely excited to add two new AEDs to our market-leading portfolio of automated external defibrillators,” said A. Ernest Whiton, President of ZOLL’s global Resuscitation division. “With the addition of these two new models, our company offers an even more comprehensive line of AEDs to meet the needs of both our public access and BLS customers, including the latest tools to better manage their AEDs’ readiness and access to clinical event data.”
“This next generation of AEDs extends ZOLL’s reputation for defibrillators that offer Real CPR Help, real-time CPR feedback, providing even better support for rescuers,” said Richard Knell-Moore, Country Manager of ZOLL Medical UK “We are pleased to make these lifesaving devices available to both lay and professional rescuers in the UK. ZOLL estimates there are at least 1 million deaths globally each year from sudden cardiac arrest.1 In the UK, the annual number of such deaths is approximately 100,000.”
About Sudden Cardiac Arrest (SCA)
SCA is an abrupt disruption of the heart’s function, which causes a lack of blood flow to vital organs. It is the leading cause of unexpected death in the world and strikes without warning. When sudden cardiac arrest occurs, the fact is that only half of the victims will need a shock. But all will require CPR. Survival is poor in most countries, typically less than 8%; improvements in resuscitation practices could save as many as half of these victims.
ZOLL AED 3: Easy to Use, Easy to Own, Easy to Maintain
The ZOLL AED 3 is easy to use because full-colour rescue images illustrate the resuscitation process. Real CPR Help guides rescuers to deliver Guidelines-compliant compressions based on the UK and European Resuscitation Council’s 2015 Guidelines for emergency cardiac care. The CPR Uni-padz™ electrodes and battery last five years, keeping the cost of ownership low and making the ZOLL AED 3 easy to own.
State-of-the-art technology makes the ZOLL AED 3 easy to maintain. It features Programme Management Onboard, which instills confidence that the AED will be ready to go when needed because it enables users to cloud-connect the ZOLL AED 3 to ZOLL’s PlusTrac™ AED Programme Management System. PlusTrac immediately notifies users by email after any failed self-test and provides alerts as the battery nears the end of its useful life.
ZOLL AED 3 BLS: The AED for First Responders
Designed specifically for professional rescuers, the ZOLL AED 3 BLS is built on a defibrillation platform of unmatched support. The CPR Dashboard shows elapsed time since power on, remaining time countdown for the current CPR cycle, number of shocks delivered, the patient’s current ECG in real time, and the depth and rate of chest compressions. For a paediatric rescue, the rescuer just has to push the Child button. The AED 3 BLS is the only AED to provide paediatric CPR metrics using the CPR Dashboard.
Providing a shock in 8 seconds with a new battery, the ZOLL AED 3 BLS is among the fastest AEDs at delivering a shock after chest compressions stop, and once the analysis starts. Because it is WiFi-enabled, first responders can easily export clinical event data to RescueNet® CaseReview, a ZOLL Online product that simplifies clinical event reporting and the viewing and distribution of CPR quality metric analysis.
ZOLL AED 3 units are available in many languages, including English, French, Spanish, German, Italian, Dutch, Swedish, Latin American Spanish, and Danish.
ZOLL AED 3 units are not available for sale in the United States or Canada. These products are pending regulatory approval from the U.S. Food and Drug Administration or Health Canada.
About ZOLL Medical Corporation
ZOLL Medical Corporation, an Asahi Kasei Group Company, develops and markets medical devices and software solutions that help advance emergency care and save lives, while increasing clinical and operational efficiencies. With products for defibrillation and monitoring, circulation and CPR feedback, data management, therapeutic temperature management, and ventilation, ZOLL provides a comprehensive set of technologies that help clinicians, EMS and fire professionals, and lay rescuers treat victims needing resuscitation and acute critical care. For more information, visit www.zoll.com.
About Asahi Kasei
The Asahi Kasei Group is a diversified group of companies led by holding company Asahi Kasei Corp., with operations in the material, homes, and health care business sectors. Its health care operations include devices and systems for acute critical care, dialysis, therapeutic apheresis, transfusion, and manufacture of biotherapeutics, as well as pharmaceuticals and diagnostic reagents. With more than 30,000 employees around the world, the Asahi Kasei Group serves customers in more than 100 countries. For more information, visit www.asahi-kasei.co.jp/asahi/en/.


